Aseptic Fill & Finish Services for Small Batches in Clinical Phases
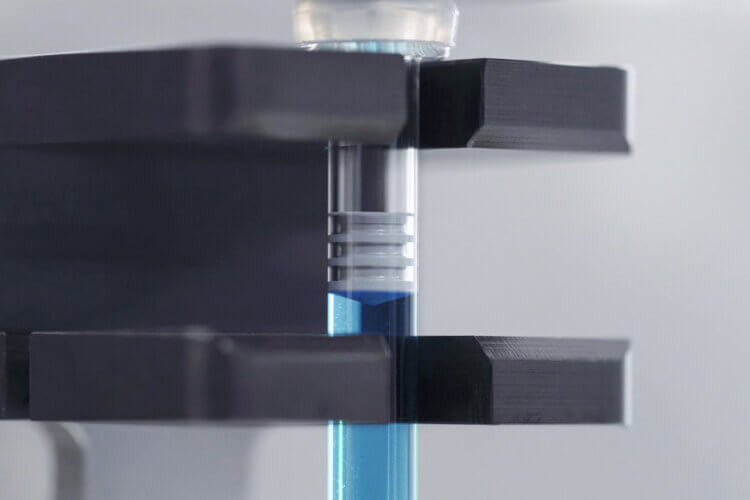
In the area of clinical phases, we offer contract filling of small batches under LF as well as in safety workbenches, either manually or with highly flexible semi-automatic filling and closing equipment from groninger and Berner (safety) workbenches.
These processes, mainly for filling vials in phases 1 and 2, offer our customers the greatest possible flexibility and an enormous time advantage. Hereby, we support our customers with sterile filling (GMP and non-GMP) – including engineering batches, stability tests as well as verification of container closure integrity.
For phase 3, we offer aseptic filling for glass or polymer vials, syringes or cartridges. The filling processes developed and applied in the clinical phases, including all common gassing and vacuum options, can subsequently be scaled up to our commercial groninger production lines through a simple, cost-effective and fast technology transfer.
Our capabilities at the Pharmacampus include aseptic processing for “Small & Large Molecules”, but also the processing of “mRNA”, “Cell & Gene” products such as viral vectors (AAV) under S2 conditions or toxic products up to OEB 5.
Project enquiries to Thorsten Häfner
E-mail: thorsten.haefner@psm-saar.de
Phone: +49 172 3213362



Our End-To-End CDMO Services for Clinical Phases
- Manufacturing and filling processes for engineering & feasability studies, technical trials, sterility testing and stability studies as well as clinical trials under GMP conditions
- Manual or semi-automated filling, stoppering and closing of pre-fillable syringes, vials, cartridges and customized container types for small batches
- Analytical services for product development, stability studies as well as product release
- Manual or fully automated visual inspection, labelling and packaging
- Storage and shipping of products at ambient temperature, cooled at 2° – 8° or frozen down to -75° C.