Your Pharma CDMO for Aseptic Fill and Finish Services for PFS, Vials, and Cartridges
PSM GmbH is a family-run German company that operates as a Contract Development and Manufacturing Organization (CDMO) in the pharmaceutical industry, located in Schiffweiler in the state of Saarland. With our new state-of-the-art facility, we are proud to be one of the first European CDMOs to have passed the inspection by the local authorities under the new EU GMP Annex 1.
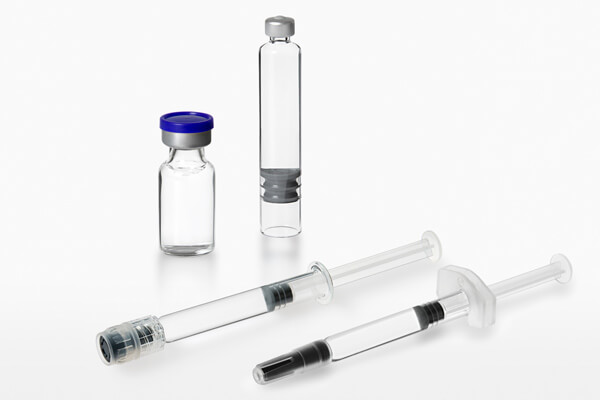
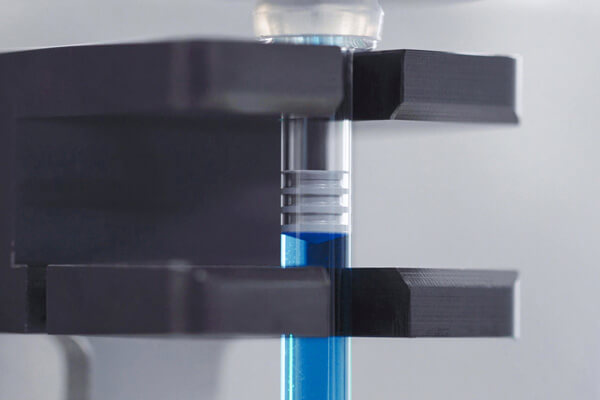
As an end-to-end CDMO, we provide services for the entire implementation process and lifecycle of your drug, from clinical phases to commercial GMP manufacturing. Our portfolio includes method and process development, in-house analytical services, aseptic fill and finish services, visual inspection with CCIT, secondary packaging with serialization, as well as GMP storage and GDP transport.
Our Services as a Pharma End-to-End CDMO