Analytical Services
We have a profound knowledge, state-of-theart labs, as well as the technical equipment at PSM to perform the necessary analytics within the scope of drug development and drug manufacturing for products in the clinical phases, as
well as commercial products. The product release is performed by our own
QP staff at PSM. We also offer these analytical services as part of contract services for products that are not developed or filled in-house.
Project enquiries to Thorsten Häfner
E-mail: thorsten.haefner@psm-saar.de
Phone: +49 172 3213362


Our Service Portfolio Includes the Following Items:
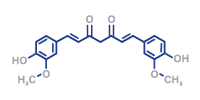
- HPLC (UV/ VIS, DAD)
- Particle size detection
- UV-VIS
- Water content
- Titration
- IR spectroscopie (FT-IR)
- Viscosity
- Polarimetry
- Stability studies
- LC-MS/MS (non-GMP)
- Elemental impurities assessment and control
- Refractrometry
- Tests according to USP/Ph. Eur.
- Identity verification acc. to Ph. Eur:
– identification
– purity/assay - In-vitro drug release (Apparatus II)
- Further analytics like microbiology, residual solvents or elemental impurities will be performed by our qualified partner laboratories.