Why curcumin?
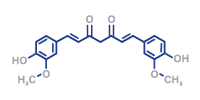
Curcumin has a broad therapeutic effect on the human body, as it affects different molecular targets and thus influences various metabolic pathways. Possible therapeutic approaches of curcurmin are complex and diverse and have not yet been completely explored in their complexity. Its antioxidative, anti-inflammatory and anticarcinogenic potential is of great interest. Curcumin is able to regulate both antioxidative and prooxidative processes. The antioxidative characteristics are determined by the chemical structure of curcumin.
Among other things, curcumin has the ability to intercept radicals and protects from oxidative damage. In the treatment of tumors, the pro-oxidative characteristics lead to a triggering of planned cell death in degenerated cells; an effect that does not happen in healthy tissue. However, the anti-inflammatory, antioxidative properties and the influence on cell proliferation also contribute to the anticarcinogenic effect.

Purecuma® is the purest curcumin

Is a registered trademark (No. 30 2020 203 011)
Our manufacturing process:
Purecuma® is the purest curcumin (purity > 92 %), produced according to GMP standards.
The quality testing of Purecuma® is carried out in the Quality Control Department of PSM GmbH, where regular quality checks of pharmaceutical raw materials and finished drugs are carried out at the highest level.
Each batch of Purecuma® is made and inspected for purity and content according to well-established and validated manufacturing instructions, analytical methods and legal requirements. Only the most modern, regularly maintained and qualified, state-of-the-art equipment is used to manufacture and test Purecuma® products, in order to ensure the reliability of the data obtained. The highly qualified employees are continuously trained through internal and external training and further qualification. Regular GMP inspections certify that PSM GmbH complies with the GMP guidelines.
Regular quality control ensures that only curcumin that meets the highest quality standards is approved. Purecuma® is therefore suitable as an active ingredient for all possible areas of application; from topical, through oral to parenteral – Purecuma® is available for a wide range of therapeutic possibilities.
Purecuma® is distributed and by PSM GmbH, if you are interested please contact Thorsten Häfner.


Highest purity of the active ingredient curcumin (> 92 %,
< 10 % desmethoxycurcumin,
< 1 % bidesmethoxycurcumin)

Valid certificate of analysis

Pharmaceutical quality, suitable for parenteral use

Quality control according to “Good Manufacturing Practice (GMP)”

Effectiveness tested in vitro


Usually mixtures of ground roots (only 70-80 % curcumin)

Food supplement, only suitable for oral application

Usually only visual inspection