Your End-to-End Pharma CDMO in Germany
PSM GmbH is a family-run German company that operates as a Contract Development and Manufacturing Organization (CDMO) in the pharmaceutical industry. Our facility is located in Schiffweiler, Saarland, near by the historic “Grube Reden” – a former coal mine in Landsweiler-Reden. With around 100 employees and state-of-the-art equipment, we manufacture pharmaceutical products in Germany, making a vital contribution to ensuring the availability of essential drugs for people.
We are committed to providing the best possible support to patients and enhance their quality of life. To achieve this, we focus on the highest pharmaceutical quality, modern fill & finish technologies, and innovative processes. With passion and precision, we develop agile, GMP-compliant end-to-end solutions for aseptic contract manufacturing – reliable, efficient, and always at the forefront of technology.
With our new state-of-the-art facility, we are proud to be one of the first European CDMOs to have passed the inspection by the local authorities under the new EU GMP Annex 1.
As an end-to-end CDMO, we provide services for the entire implementation process and lifecycle of your drug, from clinical phases to commercial GMP manufacturing.
Our portfolio includes method and process development, in-house analytical services, aseptic fill and finish services, visual inspection with CCIT, secondary packaging with serialization, as well as GMP storage and GDP transport.
In order to support patients and improve their quality of life, we put passion into our daily work, focus on pharmaceutical excellence and use the latest fill and finish technologies and processes. We offer the most advanced, agile and, above all, GMP-compliant end-to-end solutions for aseptic contract manufacturing.

1960 – 2022
Viktoria Apotheke
1960: The Trennheuser family acquires the Viktoria Apotheke in Saarbrücken.
1997: The Viktoria Apotheke begins under the direction of Dr. Fritz Trennheuser with the manufacturing of sterile formulations. The business segment will be continuously expanded in the coming years with great commitment.

2007
Topmedicare GmbH
2010: Topmedicare acquisition into a group of companies with Viktoria Apotheke. In addition, Topmedicare takes over the distribution of Trennheuser Cosmetics.
2016: Contract manufacturing
Topmedicare in Saarbrücken is a contract manufacturer according to § 13 AMG for the manufacturing of sterile products for IMPs and small batches as well as non-sterile medicinal products and clinical investigational medicinal products according to the highest quality criteria of the GMP. From the synergies between the manufacturing company and pharmaceutical competence acquired over decades, joint products are optimally implemented.
Visit the homepage of Topmedicare GmbH: www.topmedicare.de

2020
PSM GmbH – Your Pharma CDMO for Aseptic Fill & Finish Services
Founding of PSM GmbH in Schiffweiler and registration in the commercial register.

2021-2024
Construction of PSM GmbH in Schiffweiler
The construction of the facility for PSM GmbH involves the development of a state-of-the-art pharmaceutical production site, which will include approximately 1,500 sqm of cleanroom space, 1,000 sqm of laboratory space, and 1,000 sqm of office space.

2024-2025
PSM GmbH starts
PSM launches pharmaceutical services of the highest quality and receives the GMP certificate in 2024 for the manufacturing of pharmaceutical products in accordance with §13 AMG.
The PharmaCampus in Schiffweiler is a company alliance
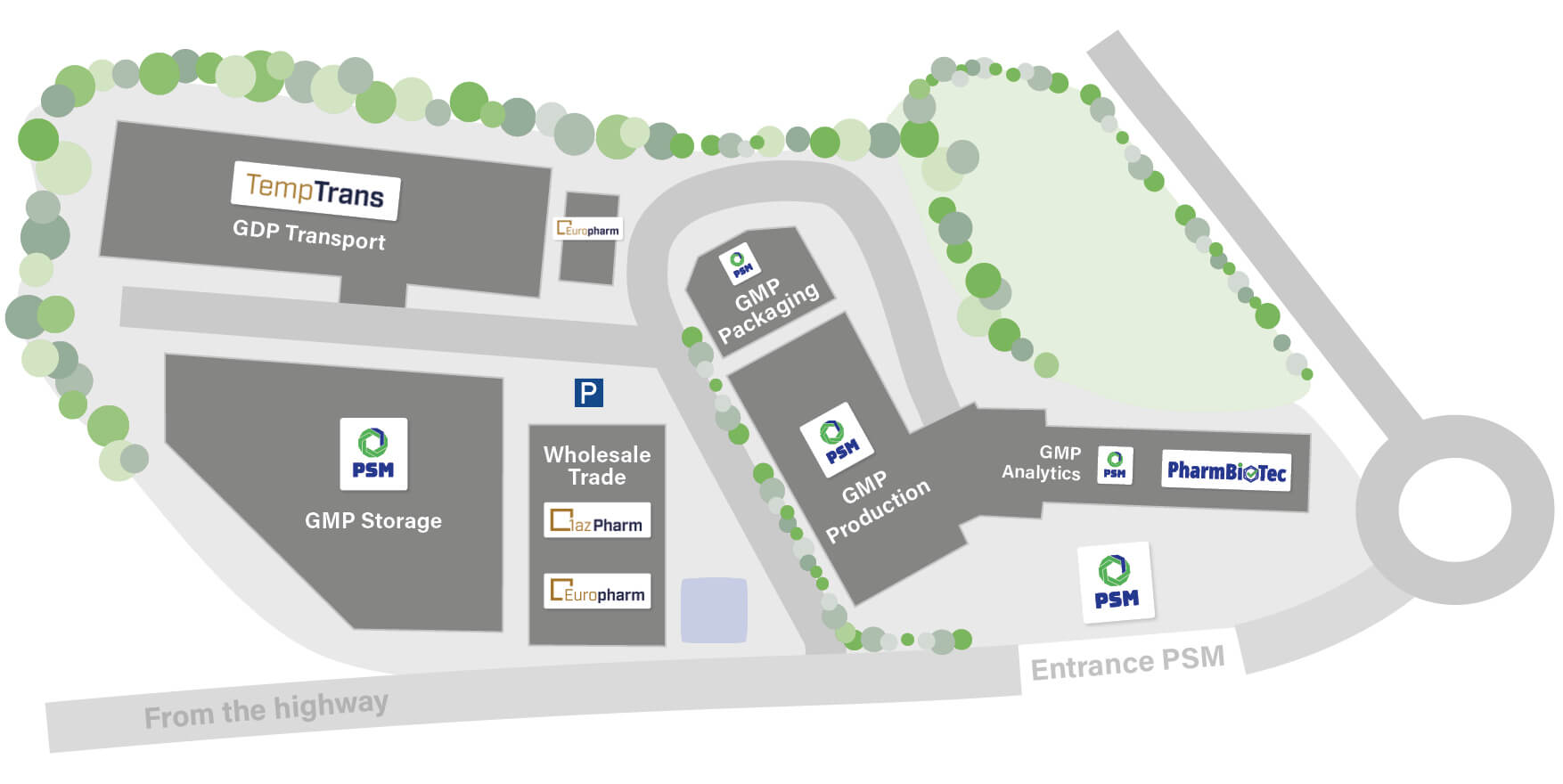
We offer you customized solutions throughout the development cycle of your pharmaceutical product
PSM GmbH likes to support its customers as a full-service provider throughout the entire development cycle of a medicinal product – from product development down to shipping. With us, you get everything from one source.


