PSM GmbH – CDMO for Pharmaceutical Service
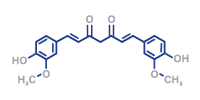
The PSM GmbH is a German Contract Development Manufacturing Organization (CDMO) our services include api manufacturing, product and process development and small to mid-size aseptic gmp fill and finish services. Furthermore, our service includes everything from formulation development through production and analytics to market release. Our product portfolio also includes the synthesis of active ingredients.
GMP, or Good Manufacturing Practice, is a set of regulations enforced by regulatory agencies worldwide to ensure the quality, safety, and efficacy of pharmaceutical products. For gmp pharma companies, compliance with GMP is not only a regulatory requirement but also a commitment to producing safe and effective medications.